Research Activities
A2. Photocatalytic processes with TiO2 nanostructured materials
A. Catalytic-photocatalytic reactions for environmental detoxification (Advanced Oxidation Processes) and synthesis of nanostructured materials with environmentally friendly methods
Metal oxides, mainly TiO2, and polyoxometallates (POM) mainly of W, have been used in thermal and mainly photochemical reactions according to the following photocatalytic cycle:

A. Hiskia, A. Mylonas and E. Papaconstantinou, Chemical Society Reviews, 2001, 30, 62-69
[top of page]
A1. Photocatalytic processes with POM
Representative structures and spectra of POM are shown below:
|

|

|

|
|
PW12O403-, Keggin Structure
|
P2W18O626-, Dawson Structure
|
The stucture of isopoly W10O324-
|
|

|
|
Oxidized (a) and reduced (b) by one and two electrons spectra of PW12O403-
|
E. Papaconstantinou, Chemical Society Reviews, 1989, 18, 1-31
POM upon illumination at the O → M CT band (i.e., in UV and near-visible area) become powerful oxidizing reagents able to oxidize various organic compounds. In the process POM undergo stepwise reduction accumulating electrons that can be subsequently delivered, via thermal reactions to a variety of oxidants. This way a great variety of organic compounds are oxidized and indeed mineralized to CO2, H2O and inorganic anions and several organic and inorganic compounds can be reduced via a photocatalytic process in which POM serve as electron relays. Thus, in principle, POM can serve as decontamination photocatalysts of aquatic media removing both organic pollutants and metal ions. Under special conditions controlled photocatalytic reactions have been reported. In addition, the reduction-precipitation of metal ions mediated by POM may lead to formation of metal nanoparticles in which POM serve as reducing reagents and stabilizers. Our main contribution has been with the use of POM in the following areas, (representative references are given in parentheses):
[top of page]
(a) Non-selective oxidation (photodegradation) of organic pollutants to CO2, H2O and inorganic anions
The above method has been applied to many organic pollutants, such as phenol, chlorophenols and chloroacetic acids [1, 2, 3, 4, 5]. OH radicals generated by reaction of POM with H2O seem to play a key role in the process. Dioxygen is important in that it oxidizes (regenerates) the catalyst and through reductive activation may or may not participate further in the process, depending on the substrate [6]. Due to their photocatalytic performance, POM can be recognized as a new AOP. Recently, the photocatalytic degradation of some diversified pesticides has been performed. Among the target pesticides was lindane [7], a typical organochlorine insecticide with a very high stability in the environment, the organophosphorous insecticide fenitrothion [8], the herbicide atrazine [9], and the acid herbicide, bentazone [10]. In all cases, the final degradation products were CO2, H2O, and inorganic anions, with the exception of atrazine, which is degraded into the non-toxic but persistent cyanuric acid. Several intermediates have formed prior to mineralization giving rise to complicated degradation mechanisms. As an example, the intermediates detected in case of fenitrothion are presented in the following scheme.
|

|
|
Intermediates formed during the photocatalytic degradation of fenitrothion by H3PW12O40 or TiO2
|
P. Kormali et al., Appl. Catal.: B: Environmental, 2004, 48(3), 175-183
POM are at least as effective as the widely published TiO2. Both systems have been compared in parallel experiments and there has been demonstrated their similarity in terms of the overall photocatalytic performance, intermediates and final degradation products [8].
[top of page]
(b) Reduction-removal of metallic ions
Recently, a novel photocatalytic method for the selective reduction and recovery of several metal ions from their aqueous solutions based on POM has been reported. By suitable choice of POM and organic substrate, several metal ions (Ag+, Cu2+, Pd2+, Au3+, Hg2+, Ni2+, Cr6+), can be reduced to a lower or zero oxidation states. This way, photocatalytic decontamination of aqueous solutions, from organic pollutants and metal ions can take place, upon illumination in the presence of POM [1, 2, 3, 4, 5, 6].
[top of page]
(c) Reductive decomposition of organic compounds
In a photocatalytic mode, the excited POM, produced after absorption of UV-near visible light, is a strong oxidant capable of mineralizating a variety of organic species, including organic pollutants to CO2 and inorganic anions. Reduced POM can deliver their electrons to great variety of chemical species, such as O2, H+, nitroaromatics and metal anions among others. Resently, POM (PW12O403- and SiW12O404-) are proposed as efficient photo-catalysts for the reductive degradation of the azo dyes Acid Orange 7 (AO), Naphthol Blue Black (NB) and Disperse Blue 79 in aqueous or ethanol solutions [1, 2, 3]. The process involves absorption of light by polyoxometalates, oxidation of an organic sacrificial electron donor, for instance propan-2-ol or ethanol, and the subsequent reductive destruction of the azo dye by reduced POM with the concomitant recycling of the POM catalyst to the oxidized form. Results show that the dyes are reduced to aromatic amine derivatives. On the other hand, photooxidative decomposition of azo dyes, that also takes place, involves again absorption of light by POM followed by direct or OH-mediated oxidation of the dye. This process is an order of magnitude slower than reductive elimination, but leads to mineralization of the azo dye [3].
[top of page]
(d) Controlled size synthesis of metal nanoparticles
We have recently shown that by keeping the ionic strength low, illumination of a (Substrate/POM/Mn+) solution leads to formation of metal nanoparticles, through a process in which POM serve both as photocatalysts-reducing reagents and stabilizers [1, 2, 3].
According to the following reactions:
POM + S ------> POM(e-) + Sox
POM(e-) + Mn+ ---------> POM + M0coll
(S = organic substrate, Mn+ = metal ion)
metal nanoparticles with average diameter less than ca 15 nm and small size distribution (< 25%) have been formed from Ag+, Pd2+, Au3+ and Pt4+.
The process exhibits both practical and academic interest. From the practical point of view, it involves an environmental friendly and simple way to synthesize metal nanoparticles since: (a) It takes place at room temperature, rapidly, within a few seconds, utilizing a mild reductant, i.e. SiW12O405– (0.057 V vs. NHE) in catalytic amounts. On the other hand, other reducing methods that proceed at room temperature need extremely strong reductants such as BH4 –, hydrogen atoms, solvated electrons or organic radicals that are formed upon radiolysis with γ-rays or sonolysis of aqueous solutions, while other methods that utilize mild reductants request high-stoichiometric amounts and heat in order to complete even after hours or days. (b) The synthesis of metal nanoparticles is achieved in water, a non-toxic solvent. (c) POM, besides reductant, serves also as stabilizer, avoiding the addition of a second, sometimes toxic, reagent that would be needed to arrest the metal particles in nano-dimensions.
The fact that the above reactions can be separated in time and space makes size selectivity of metal nanoparticles to be achieved on the basis of rate control of Mn+ reduction. The unique ability of POM to exhibit widely varied and precisely controlled redox potentials depending on intrinsic properties could extent the applications of this size selective process [4].
Verification of our previous studies according to which POM serve as both reducing reagents and stabilizers in synthesis of metal nanoparticles has been achieved by characterization of nanoparticles using high resolution transmission electron microscopy (HRTEM) [5]. The figure below is a representative HRTEM image of one Se0 crystal nanoparticle produced upon photolysis of SiW12O404- /propa-2-ol to form one-equivalent tungstate, SiW12O405- and addition of Se(IV). An amorphous shell, 1–3 nm thick, surrounds the Se crystalline core.
This shell was studied by energy filtered transmission electron microscopy (EFTEM). The EFTEM images represent elemental maps, where a bright area indicates a high elemental concentration. The images presented below are evidence that the core of the nanoparticles is Se, while the surrounding shell is composed of O, Si and W, that is, POM.
|

|
|
(a) Bright field image and (b) Se, (c) O, (d) Si and (e) W EFTEM images of a Se0 nanoparticle
|
T. Triantis et al., Catalysis Today, 2009, 144, 2-6.
[top of page]
(e) Inorganic-Organic Multilayer Films based on Polyoxometalates
Most of the studies on POM have focused on homogeneous solutions. Although homogeneous decontamination is a useful process, POM present a disadvantage for the recovery of catalyst, which is necessary in many cases. Therefore immobilization of these catalysts is very important. In this frame, original photochromic and photocatalytic multilayer films are produced by the polyoxometalate (POM) SiW12O404- and poly(ethylenimine), via the layer-by-layer (LbL) self-assembly method. The resulting multilayer films have been characterized by UV-Vis spectroscopy, FTIR and atomic force microscopy (AFM) [1]. Irradiation with ultraviolet-near visible light of this composite material causes reduction to POM (SiW12O405-), while the films acquire the characteristic blue color of reduced POM. Oxygen plays a great part in the gradual reoxidation of the coated POM, resulting in the reversible photochromism of the films.
|
|
|
|
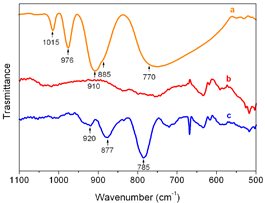
FTIR spectra of (a) H4SiW12O40, (b) a film with one PEI layer and (c) a (PEI/SiW12O404-)7 multilayer film fabricated on silicon wafer substrate
|
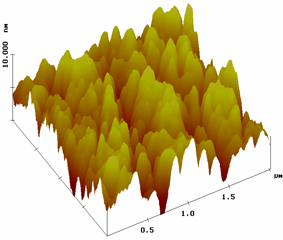
AFM image of a PEI/SiW12O404- film on quartz slide
|
T. Triantis et al., JAOT, 2008, 11, 231-237
[top of page]
(f) Previous research activities and contribution on POM photocatalysis
- Photochemistry of polyoxometallates [1, 2, 3, 4, 5]
- Water splitting (hydrogen production) [6]
- Photoelectrochemical production of electricity [7]
- Modification of electrodes (photoelectrochemical reactions) [8]
- Selective oxidation-synthesis of organic chemicals [9, 10]
A2. Photocatalytic processes with TiO2 nanostructured materials
(a) Photodegradation of organic pollutants by TiO2 .Elucidation of the reaction mechanism
The photocatalytic degradation of bromomethanes (CBr4, CHBr3 and CH2Br2) and halomethanes containing chlorine and bromine (CCl3Br, CHCl2Br, CH2ClBr) or bromine and fluorine (CBr3F) over irradiated TiO2 has been investigated under aerobic and anaerobic conditions. For all of those compounds, the complete disappearance of the primary compound and the stoichiometric concentration of halides was achieved. Degradation proceeds through combined reductive and oxidative processes [1, 2].
|
The photocatalytic degradation of the organophosphorus insecticide diazinon in aqueous suspensions has been studied by using TiO2. The degradation of the insecticide was a fast process and included the formation of several intermediates that were identified using GC/ion-trap mass spectrometry with EI or CI in positive and negative ionization mode and HPLC/electrospray-QqTOF mass spectrometry. Since primarily hydroxyl derivatives were identified in these aqueous suspensions, the mechanism of degradation was probably based on hydroxyl radical attack [3].
Recently, in the frame of the European Project “CleanWater” highly efficient N- and N-F- doped TiO2 nanoparticles with enhanced structural properties and high photocatalytic activity under visible light irradiation have been used for the degradation of microcystin-LR (MC-LR), geosmin (GSM) and 2-methyl-isoborneol (2-MIB) (unpublished results).
|
 |
[top of page]
(b) Comparison of the photoredox properties of polyoxometallates and semiconducting particles
We have demonstrated that two large categories of compounds, namely, polyoxometallates and aggregates of various metal oxides have similar properties. Emphasis was given to the photocatalytic properties of the two systems in terms of the overall mechanism of photodecomposition of organic compounds, the intermediate species involved and the final photodegradation products (i.e., CO2, H2O and inorganic anions) [1]. The similarity of behavior has been attributed to the formation of the common powerful oxidizing reagent, OH radical, from the reaction of the excited catalyst and water molecules. Further studies provide substantial evidence that the apparent photooxidation mechanism of these two categories of photocatalysts is circumstantial, depending on substrate and the mode of investigation. Overall, though, the action of OH radicals relative to h+ appears to be more pronounced with PW12O403- than TiO2 [2].
[top of page]
B. Environmental Analytical Chemistry
Development of Methods for the determination of toxic pollutants in trace level in water, foodstuff and environmental samples
Contamination of water supplies with organic pollutants such as PAHs, PCBs, pesticides and hazards of biogenic origin, i.e., cyanotoxins is one of the most important global problems. Recent EU Directives propose the determination of these target pollutants in drinking and surface water and set their maximum concentration. Resulting from the above, it is mandatory to monitor these analytes using appropriate methods. The development of new advanced analytical methods can give rise to sensitive and reliable determinations even at the ppt level. Our contribution to this area of research can be summarized into the following:
[top of page]
(a) Development of Methods for the determination of cyanotoxins in surface and drinking water by using SPE and LC/MS-MS
Cyanobacteria (blue-green algae) are considered an important water quality problem, since several genera can produce toxins, known as cyanotoxins that are harmful to human health. Blooms of toxic cyanobacteria have been reported worldwide and several incidents of wild and domestic animal poisoning as well as human injury and death have been reported. Hepatotoxic cyclic peptide toxins (microcystins, MCs, and nodularins) are the most widespread cyanotoxins that are present in diverse environments [1]. MCs are cyclic heptapeptides that are produced by different cyanobacteria genera such as Microcystis, Anabaena, Plankothrix and Nostoc. About 80 MC variants have been identified so far in natural water samples and cyanobacterial cultures, with microcystin-LR (MC-LR) being the most common and most toxic variant. Nodularins are similar toxic cyclic pentapeptides that are produced by different cyanobacterial strains of the genera Nodularia whereas motuporin, a structural analog of nodularin, was isolated from a marine sponge. During the last years there is a growing concern regarding the health effects of MCs and nodularins, because they act as tumor promoters, through the inhibition of protein phosphatases 1 and 2A, which play a key role in cell regulation. In response to this concern, WHO has recommended for provisional adoption the value of 1 μg/L as a Guideline Value for MC-LR concentration in drinking water. This has resulted to the need for development of reliable analytical methods for the monitoring of this class of toxins in water supplies on a quantitative basis.
Our contribution was focused on the development of a system of analytical methods that addresses large scale monitoring of MCs (MC-RR, MC-YR, MC-LR, MC-LA) and NOD in surface and drinking waters. To accomplish this goal, ELISA and PPIA were used for primary quantitative screening of the target compounds, while HPLC/PDA and LC/MS/MS methods were applied to positive samples. The methods employed were validated in-house and method performance parameters were estimated. In addition, a protocol of analysis of unknown samples was developed, that uses the above methods, with the aim to serve as a cost-effective system for the analysis of large numbers of water samples. The validity of this system was tested through analysis of a number of real surface and drinking water samples. For the first time, the fitness for purpose of this analytical protocol has been discussed, with respect to analytical performance as well as time and cost of analysis [2].
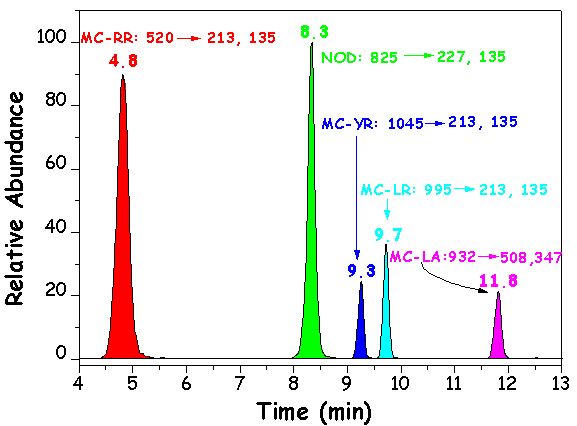
LC/MS/MS SRM chromatograms of a standard solution of MC-RR, MC-YR, MC-LR, MC-LA and NOD (20 mg/L)
T. Triantis et al., Toxicon, 2010, 55, 979-989
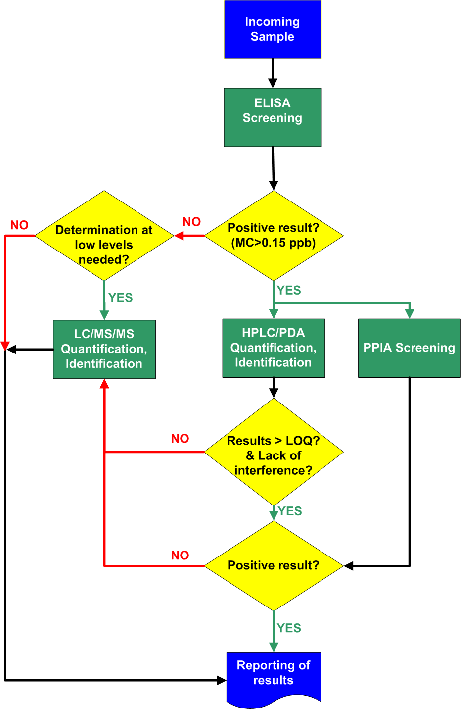
Analytical protocol for the monitoring of MCs and NOD in surface and drinking water
T. Triantis et al., Toxicon, 2010, 55, 979-989
In the area of research on identification of cyanotoxins, a fast and selective method for the sensitive determination of anatoxin-a in lake waters using LC/MS-MS and phenylalanine-d5 as internal standard, has been developed [3].
Anatoxin-a (AN) is an alkaloid neurotoxin, produced by the freshwater blue-green algae genera Anabaena, Oscillatoria, Aphanizomenon, Cylindrospermum, Microcystis, Oscillatoria, Planktothrix and Raphidiopsis. Massive proliferation of neurotoxic cyanobacteria producing anatoxin-a in waterbodies around the world has resulted in fatal intoxications of several wild and domestic animals with significant environmental and economic consequences. Τhe Department of Health of Washington State recommends the use of 1 μg L −1 as a provisional value in order to provide an adequate margin of drinking water safety.Phenylalanine is a natural amino acid, also present in freshwaters, isobaric to anatoxin-a, with a very similar fragmentation pattern and LC retention. Since misidentification of phenylalanine as anatoxin-a has been reported in forensic investigations, special care must be taken in order to selectively determine traces of anatoxin-a in the presence of naturally occurring phenylalanine. In the method developed a 1.8 μm 50×2.1 mm C18 column was used for the separation of anatoxin-a and phenylalanine, achieving a 3-min analysis time. Isotopically labelled phenylalanine- d5 was employed as internal standard to compensate for electrospray ion suppression and sample preconcentration losses. Both compounds were preconcentrated 1,000-fold on a porous graphitic carbon SPE cartridge after adjustment of sample pH to 10.5. The method was validated by using spiked lake water samples. Anatoxin-a recovery ranged from 73 to 97%, and method LOD and LOQ were 0.65 and 1.96 ng L −1 respectively [ 3]. 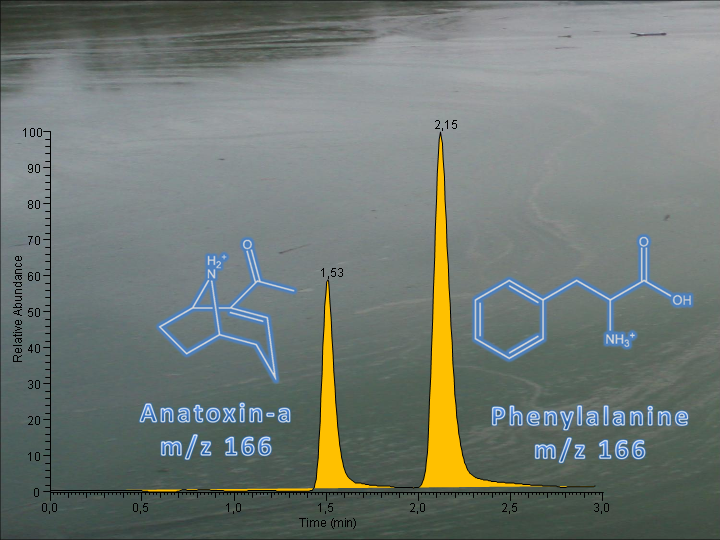 [top of page]
(b) Determination of organic halides in foodstuff (honey) by Solid Phase Microextraction (SPME) in combination with GC/ECD and GC/MS
Development of a rapid and sensitive method for the simultaneous determination of 1,2-Dibromoethane (1,2-DBE), 1,4-Dichlorobenzene (1,4-DCB) and Naphthalene residues in honey using HS-SPME coupled with GC-MS [1].
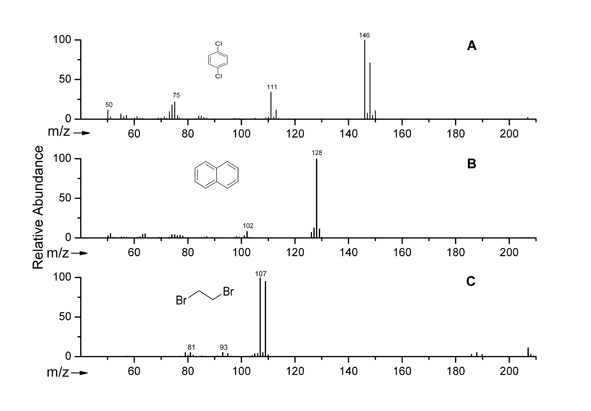
Ion mass spectra of p-DCB (A) , Naphthalene (B) and 1,2-DBE (C)
K. Tsimeli et. al., Anal. Chim. Acta, 2008, 617, 64-71
[top of page]
(c) Development and validation of a new analytical method for high sensitivity analysis of European Union 8 priority pollutant PAHs in surface and drinking water by LC/APPI/MS-MS
The developed method for PAHs determination using LC/APCI-APPI/MS-MS (unpublished results) fulfils established criteria imposed by recent legislation on environmental quality standards in the field of water policy. Various APCI and/or APPI conditions were tested and various dopants were examined in order to achieve best ionization of the selected polycyclic aromatic hydrocarbons (PAHs) (naphthalene (Naphth), anthracene, (Anthr) fluoranthene, (Fluor) benzo(b)fluoranthene (B(b)F), benzo(k)fluoranthene (B(k)F, benzo(a)pyrene, (B(a)P) benzo(g,h,i)perylene (B(g,h,i)P) and indeno(1,2,3-c,d)pyrene (Indeno)). The APCI-APPI/MS–MS operating conditions were optimized on the basis of mass spectral data and two specific transitions per compound were selected for simultaneous monitoring in SRM mode. The procedure, based on SPE extraction before analysis by LC/MS–MS, was fully validated on the basis of international protocols. Recovery and within-laboratory reproducibility for all target compounds studied were satisfactory. Low LODs were established with this method for the majority of PAHs. The proposed method proved to be a valuable tool for assessment of the occurrence of PAHs in surface and potable water samples and can be adapted for routine application in monitoring studies and surveys.
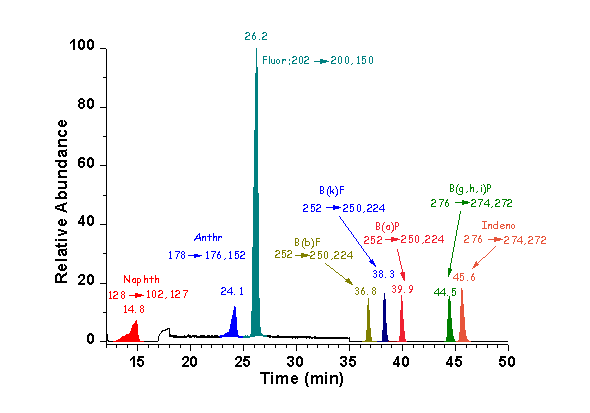
LC-MS/MS SRM chromatograms of a standard solution of the eight PAHs (100 μg L-1 for the six PAHs (Anthr, B(b)F, B(k)F, B(a)P, B(g,h,i)P) and 1000 μg L-1 for Naphth, Fluor)
(d) Previous research activities and contribution on environmental analytical chemistry
Determination of pesticides, PCBs, pharmaceuticals etc in water, foodstuff and environmental samples [1, 2, 3, 4, 5, 6, 7, 8].
[top of page]
Funded Projects
Research Projects
1. "CYANOWATER- Cyanotoxins in Fresh Waters, Advances in Analysis, Occurrence and Treatment", 2012-2015, General Secretariat for Research and Technology (GSRT) - Greek Ministry of Education, Action “EXCELLENCE” (ARISTEIA I), 350 Κ€ (Project Coordinator: Dr. A. Hiskia). This project has been successfully evaluated in a peer review process and classified between 15 (out of 144) more outstanding projects for their highest quality and scientific excellence in the field of Energy and Environment. The main objective of the Action “EXCELLENCE” is to support highly talented researchers, working in Greece, who have compiled an outstanding record in their respective fields and have the potential to advance the frontiers of scientific knowledge and scholarship. CYANOWATER project aims in filling research gaps and achieving breakthrough results in (a) Development of advanced analytical methods for emerging cyanotoxins (CTs) and for simultaneous analysis of different groups of CTs, (b) Identification of the toxin-producing cyanobacteria species in freshwater bodies and (c) Development of novel advanced oxidation processes for the detoxification of water contaminated with CTs. The strong ties and close collaboration of the CYANOWATER research team (Headed by Dr. A. Hiskia as Principal Investigator) with leading research partners and water supply companies will permit technology transfer and direct and strong beneficial impacts for the international research community involved in this field but also important societal and economic impacts at a national, European and international level. An extended synopsis of the project can be found here.
2."Water Detoxification Using Innovative vi-Nanocatalysts", 2009-2012, European Union - Seventh Framework Programme, Theme [6–4] [Environment (including Climate Change) Nanosciences, Nanotechnologies, Materials and New Production Technologies – NMP], Clean Water project, Grand agreement No 227017, 117 K€.
3.“Determination of taste and odor compounds in water”, 2009-2010, Research project funded by EYDAP SA, 20 Κ€.
4."Development of a multi-residue method for the determination of pesticides in water by LC-MS/MS. Determination of cyanotoxins and pesticides in drinking and surface water", 2008 – 2010, EYDAP S.A. Contract No 08081049-3, 25 K€.
5."Development of new biomagnetic nanomaterials for medical purposes", 2006-2008, G.S.R.T, Ministry of Development, PEP Project, 9,9 K€.
6."Development of an integrated system for the monitoring of cyanotoxins in surface and treated water using combination of advanced analytical techniques", 2006-2007, G.S.R.T, Ministry of Development, PABET Project, 27 K€.
7."Photocatalysis for the mild and selective Functionalization of non-activated C-H bonds", 2004 – 2008, COST Action D29.
8."Visible light induced degradation of textile dyes using tungstate catalysts", 2003-2006, Joint Research and Technology Program with USA (Prof. P.V. Kamat), G.S.R.T, Ministry of Development, Greece, 59,85 K€.
9."Immobilization of Polyoxometalates in inert and active substrates employed as heterogeneous photocatalysts for the detoxification of aquatic systems", 2003-2005, Joint Research and Technology Program with China, G.S.R.T, Ministry of Development, Greece, 11,7 K€.
10.“Development of photocatalytic technology for the purfication of toxic wastewater using metal oxides and polyoxometalates”, 2003-2004, G.S.R.T., Ministry of Development, Greece, project “ΠΡΑΞΕ”, 44 K€.
11.“Advanced Methods for the treatment of liquid wastes and neutralization of gas pollutants”, 2000-2006. G.S.R.T., Ministry of Development, Greece, project “Human Networks for Research and Technological Education”.
12."Sonochemical and photocatalytic destruction of triazine herbicides”, 1999-2001, Joint Research and Technology Program with Germany (Prof. H. Hennig), G.S.R.T, Ministry of Development, Greece, 13 K€.
13.“Photocatalytic degradation of organic pollutants by POM and TiO2. Comparison studies”, 1999-2001, Joint Research and Technology Program with France (Dr. P. Pichat), G.S.R.T, Ministry of Development, Greece, 15 K€.
14.“Natural and Artificial Photosynthesis”, 2000-2001, G.S.R.T., Ministry of Development, Greece, project “PENED 1999”, 47 K€.
15.“Photocatalytic managent of wastewater”, 1999-2001, G.S.R.T., Ministry of Development, Greece, project EPPET ΙΙ, 174 K€.
16.“Development of decontamination technologies. Metal oxides and semiconductors in pesticides degradation”, 1999-2001, G.S.R.T., Ministry of Development, Greece, project “PENED 1999”, 73 K€.
17.“Degradation of pollutants through homogeneous and heterogeneous photocatalysis” 1997-1999, Joint Research and Technology Program with Italy (Prof. E. Pelizzetti), G.S.R.T, Ministry of Development, Greece, 55 K€.
Networks
1.«Cyanobacterial blooms and toxins in water resources: Occurrence, impacts and management», COST Action ES1105, 2012 – 2016, Grant Holder: NCSR “DEMOKRITOS”, Budget per year:~180 K€.
Over 60 partners (scientists, other professionals and companies), from 32 European countries as well as from USA, participate in this Action. The main objective of the Action is to increase, disseminate and harmonize capabilities across Europe for the risk management of cyanobacteria and cyanotoxins in water bodies, by establishing strong and synergistic links between academia, authorities, industry and citizens.
The achievement of the above objective requires inter-and multidisciplinary efforts. A strong and integrated consortium of Action has been developed and includes scientists and researchers with recognized expertise in analytical chemistry, molecular biology, aquatic/catchment ecology, toxicology and water engineering plus end-users and stakeholders (public authorities, water supply utilities, industry). This arrival of this Action is timely because new challenges in the field have appeared recently including emerging toxins and cyanobacterial species hitherto unknown in Europe, leading to the preparation of new legislation and regulations in some European countries.
2.“Polyoxometalate Chemistry for Molecular Nanoscience (PoCheMoN)”, COST Action CM1203, 2012-2016, participation in the Action as Management Committee and core group Members (Dr. A Hiskia, Dr. T. Triantis). The main objective of PoCheMoN is to accelerate POM-based Molecular Nanoscience by creating a coherent network for world-leading education and research in POM chemistry. This first overarching COST Action in this area consolidates the European POM community and promotes strategic and efficient POM research through collaboration, thereby creating a readily accessible knowledge base for the rapid uptake of POM chemistry into Molecular Nanoscience and generating breakthrough technologies through links with aligned disciplines and companies.
Oher projects
1. Services to the following companies and organizations: ETMA S.A., SHELMAN S.A., ELPE S.A. PLINIOS S.A., GLASSART S.A., EDRASOMIHANIKI S.A., ALTEK S.A., Province of Drama – Kavala – Xanthi, Water Development Department Cyprus, Institute of Nuclear Technology & Radiation Protection at NCSR “DEMOKRITOS” and Department of Biomaterials, School of Dentistry, University of Athens, Greece.
2. "Accreditation of the Environmental Analysis Laboratory", 2005-2008, Ministry of Development, Antagonistikotita, Project EPAN, 311K€.
3."Soil investigations, evaluation of the results and suggestions for the management of Olympic infrastructure at the area of Eliniko airport", 2002–2003, Services Contract No 2000SM01000024, 47 K€.
[top of page]
Research Facilities
Most of the following instrumentation and equipment have been obtained in the frame of competitive research projects:
-
Illumination apparatus equipped with different filters providing UV-A, visible or solar light (Qriel 68820 with 1000 W Xe arc lamp, Oriel 68806 Photomax with150W Xe lamp)
-
Liquid phase, continuous flow photochemical reactor
-
Gas phase photochemical reactor
-
Spectrophotometer UV/VIS/NIR (Perkin Elmer Lambda 19)
-
GC equipped with FID, ECD and TCD detectors (Hewlett Packard 5890)
-
HPLC equipped with UV-VIS and FLD detectors (Waters)
-
Gas Chromatograph / Mass spectrometer (GC-MS) (Agilent 6890A-MSD-5973)
-
Liquid chromatograph Triple Quantrapole Mass Spectrometer (LC-MS/MS) (Thermo Electron TSQ Discovery with ESI, APCI and APPI ionization probes)
-
Ion Chromatograph (Metrohm 761IC)
-
Polarographic unit (Metrohm 693VA)
-
Τotal Organic Carbon Analyzer (TOC) (Shimadzu TOC-VCPH)
-
Sample preparation equipment (SPE and SPME devices, ovens, nitrogen evaporator, rotary evaporator, ultrasound bath)
-
Ultra-pure water unit
[top of page]
|